Dr.V.Ravi, Dr.Anita Desai and Dr.S.N.Madhusudana
Department of Neurovirology
TECHNIQUES
Cell cultures
Virus growth and titration
Methods for viral antigen detection
Methods for viral antibody detection
I CELL CULTURE TECHNIQUES
Subculture of a cell line
1. Aedes albopictus C6/36 ( mosquito gut epithelium ) cell line
2. Discard supernatant fluid from a confluent monolayer of C6/36 (Aedes albopictus ) bottle cultures.
3. Add 3 to 5 ml trypsin (Pre-warmed at 37 C ) to each culture bottle.
4. Leave the bottle at room temperature for 3-5 mins.
5. Discard the trypsin.
6. Add 5ml of growth medium to the bottle and gently detach the cells from the surface with the help of a pipette.
7. Add the required amount of medium (split ratio for this cell line is 1: 5) and distribute the diluted cell suspension to fresh flasks/bottles.
8. Incubate at room temperature for 3-7 days.
http://bahankuliahkesehatan.blogspot.com/
Primary cell culture technique
Chick embryo fibroblastic culture :
The development of routine cell culture methods has reduced the importance of eggs but they are still valuable for the isolation of many important viruses and for the production of vaccines. Fertile eggs must be obtained ideally from a specific pathogen free flock, should be clean, preferably unwashed and pale shelled to simplify candling. After laying they have to be incubated for 10 days at 37° C With 40-70% humidity and good aeration and turned twice daily. After 6 days they are candled, infertile and dead eggs are discarded. On the day of culture those with satisfactory development of chorioallantioic blood vessels and showing embryonic movement are marked with pencil to indicate the limits of the air sac.
Materials required
1. Embryonated eggs preferably 10-12 days old.
2. Phosphate buffer saline (pH 7.2) .
3. 0.25% trypsin in PBS.
4. Growth medium (MEM, supplemented with 10% bovine serum)
5. Forceps, scissors, egg cup, petri dishes, filtration unit, silicone/teflon
6. Coated magnet, solution bottles and culture flasks.
Procedure:
1. Candle and select 10-11 day old eggs.
2. Place the egg in an egg cup, air sac upwards and wipe clean with spirit.
3. Break the shell with the sharp end of a sterile forceps, and lift the membrane. With a bent forceps pick up the embryo and place it in a petri dish containing PBS.
4. Dissect the embryo in the dish, remove and discard the head, limbs and viscera. Pick up the fibroblastic tissue and transfer into a wide neck bottle containing PBS.
5. Mince the tissue finely with scissors, and wash minced tissue several times in PBS to remove blood cells and debris.
6. Transfer tissue to transfusion bottle containing sterile silicone covered magnet and 50ml of 0.25 % trypsin solution and stopper securely.
7. Trypsinize on magnetic stirrer unit 37° C for 30 mins : avoid frothing of contents.
8. The tissue will disintegrate, forming a turbid suspension of cells. Filter the suspension through sterile gauze and centrifuge filtrate for 10min at 1000 rev/min.
9. Discard supernatant and resuspend cells in 100 ml growth medium.
10. Centrifuge once again and resuspend the cells in fresh medium.
11. Dilute 0.9ml suspension with 0.1ml trypan blue solution and count cells in haemocytometer.
12. Adjust concentration to 1 10 cells / ml growth medium and dispense into tubes or bottles.
13. Incubate at 37° C until monolayer is formed (2-3 days).
14. When cells have formed a monolayer, remove growth medium, inoculate virus, add maintenance medium (MEM, Eagles base with 1-2% bovine serum) and incubate.
Media preparation for cell culture
Dehydrated Tissue Culture Media
1. Take 900ml of triple distilled water.
2. Add the contents of one unit vial of dehydrated media to the water at room temperature with stirring until dissolved.
3. Rinse the vial with a small amount of triple distilled water to remove traces of powder and add to the above solution.
4. Add 2.2 grams of sodium bicarbonate or HEPES Buffer.
5. Adjust the pH if required between 7.1 to 7.4 using 1N HC1 or 1N NaOH or by bubbling carbon dioxide. Note that pH tends to rise during filtration and hence adjust it 0.2 to 0.3 units below the final desired pH.
6. Make up final volume to 1000ml with triple distilled water.
Sterilization of tissue culture Media
1. Sterilize the media by filtering through sterile membrane filter(sterilized by autoclaving at 15 lbs for 15 min & 121° C ) of 0.22 micron or less porosity using positive pressure to minimise loss of carbon dioxide.
Antibiotics
2. The following antibiotics can be aseptically added to litre of media :
3. Amphotericin 2.5 mg
4. Gentamycin (50mg/ml. Solution) 1.0 ml
5. Benzyl Penicillin 10000 units
6. Streptomycin 100 mg
Sterility check
Add 0.5 - 1.0 ml of filtered media to a tube containing sterile thioglycolate broth and incubate at 37° C for 48 hours. If the broth is clear after 48 hours the media is sterile.
TVG (Trypsin Versene Glucose)
1. TVG consists of the following components in 1X PBS:
2. Trypsin 0.1%
3. Versene 0.2%
4. Glucose 0.05%
Stock solutions for TVG
1). 10x PBS
NaCl 80.00 gms
KCl 2.00 gms
Na2H P04 14.42 gms
KH2 PO4 2.00 gms
Dist.H2O upto 1 litre
To prepare 1X PBS - add 100 ml of 1X PBS to 900 ml of D/W.
2) 2% TRYPSIN
Trypsin 2.00 gms
D/W upto 100ml
Stir the above solution on a magnetic stirrer for 4h or O/N at 4°C. Sterilize by filtering through sterile membrane filter of 0.22u pore size. A sterility check can bedone before using the solution.
3) 0.2 % VERSENE
EDTA 200 mg
D/W 100 ml
Sterilize by autoclaving at 15 lbs & 121°C for 15 minutes.
1) 10 % GLUCOSE
Glucose 10.00 gms
D/W upto 100 ml
Sterilize by autoclaving at 15 lbs & 121° C for 15 minutes.
2) 1 % PHENOL RED
Phenol Red 1.00 gm
D/W upto 100ml
Preparation of working solution of TVG
Prepare 840ml of 1X PBS and to this add 1.0 ml of 1% phenol red (indicator). Sterilize by autoclaving at 15lbs and 121°C for 15 minutes. Cool this sterile solution and then add the following sterilized solutions to it :
Trypsin 2.0% 50 ml
Versene 0.2% 100 ml
Glucose 10.0% 5 ml
Do a sterility test as mentioned above before using this TVG.
II WASHING OF GLASSWARE FOR TISSUE CULTURE WORK
1) Autoclave all glassware before washing, at 120°C, 15psi for 20min.
2) The outside surface of glassware can be cleaned with Vim and inside with only Teepol and scrubbed with a clean brush , which is only meant for brushing glassware. Wash in tap water and leave in 5% Teepol solution overnight (can be boiled)
3) Next morning wash in tap water at least 20 times and leave in 10% HC1 overnight.
4) Next day wash in tap water and rinse in 2 changes of demineralised distilled water at least 10 times in each, leave in third bucket of demineralised distilled water overnight.
5) Take out next day, dry it in hot air oven, pack and sterilize.
Packing and sterilization
Pipettes: Wrap the pipette with brown paper and sterilize in a hot air oven at 160°C for two hours.
Petridishes : Wrap the Petri dishes in brown paper and tie with twine and sterilize by hot air oven at 160°C for two hours.
Culture & media storage bottles, measuring cylinders and beakers :
Cover mouth with aluminum foil, over which brown paper is tied with twine at the neck and sterilize in a hotoven at 160°C. Plastic measuring cylinders and beakers are autoclaved at 120°C, psi for 30min.
Plastic centrifuge tubes, Eppendorf tubes, screw cap vials & tubes, :
Arrange neatly the following items in a plastic orglass beaker and cover the mouth with aluminum foil andover that wrap brown paper tied with twine at the neck.
Sterilize by autoclaving at 120°C, 15 psi for 30min.
Coverslips: Coverslips are put into a Petri dish , which is covered with brown paper and tied with twine. Sterilize in a hot air oven at 160°C for two hours.
Filter Apparatus: Wrap filter apparatus first with aluminum foil and then with brown paper, tie it with twine tightly. Sterilize by autoclaving at 120° C,
15 psi for 30min.
Solutions: Plug the mouth of the container with cotton, wrap it with brown paper and tie it with twine. Sterilize by autoclaving at 121° C , 15psi for 20 min.
I I I DEMONSTRATION OF CYTOPATHIC EFFECT ( CPE )
1) HSV - Demonstration of cytopathic effect (CPE) in Vero cell line.
2) Grow Vero cells to confluence in Nunc flasks / 24 well dishes.
3) Discard the growth medium.
4) Infect the cell line with HSV-1 suspension at an MOI (multiplicity of infection) of 1.
5) Adsorb the virus for 30 minutes at 37° C .
6) Add the maintenance medium - 5ml for Nunc flask/10-15ml for Milk dilution bottle/100ml for a Roux bottle.
7) Incubate the bottles at 37°C in CO incubator.
8) Observe daily for CPE both in the morning and in the evening.
9) HSV produces CPE by 24-48 h. Two forms of CPE are observed. The most common begins with cytoplasmic granulation after which the cells become enlarged or ballooned. These macrocytes then become rounded, take on a refractile appearance, and undergo lytic degeneration. The second type of CPE is formation of multinucleated giant cells.
Measles virus : Demonstration of CPE in Vero cell line
1) Grow Vero cells to form a monolayer in Nunc flasks/ Milk dilution bottles/Roux bottle.
2) Discard the growth medium.
3) Infect the cell line with measles virus suspension at an MOI(multiplicity of infection) of 1.
4) Adsorb the virus for 30 minutes at 37° C.
5) Add the maintenance medium - 5ml for Nunc flask/10-15ml for Milk dilution bottle/100ml for Roux bottle.
6) Incubate bottles at 37°C in CO incubator.
7) Observe daily for CPE both in the morning and in the evening.
8) Measles virus produces CPE after 4-5 days. Two types of CPE are described. One type seen after infection with wild virus and dilute inoccula is giant cell transformation. Multinucleated giant cells containing 10-100 nuclei form as a result of cell fusion induced by virus. The second type of CPE, spindle cell formation, is associated with measles virus , which has been passed repeatedly in tissue culture, or with repeated passage of undiluted inoccula.
Japanese Encephalitis virus : Demonstration of CPE in porcine stable kidney cell line (PS)
1) Grow PS cells to form a monolayer in Nunc flasks/Milk dilution bottles/Roux bottle.
2) Discard the growth medium.
3) Infect the cell line with measles virus suspension at an MOI (multiplicity of infection) of 1.
4) Adsorb the virus for 30 minutes at 37° C.
5) Add the maintenance medium - 5ml for Nunc flask/10-15ml for Milk dilution bottle/100ml for a Roux bottle.
6) Incubate the bottles at 37° C in CO incubator.
7) Observe daily for CPE both in the morning and in the evening.
8) JEV produces CPE after 18-20 hrs. CPE is seen in the form of rounding of cells followed by cell lysis.
IV VIRUS TITRATION
Titration of Rabies virus in vivo by intracerebral inoculation
After a virus is propogated in either cell culture or in a suitable animal, we need to know the infectivity titre of the virus material obtained. This can be determined in vivo by inoculating increasing dilutions of the virus material to a susceptible host animal such as laboratory mice and based on mortality seen in different dilutions, the infectivity titre which is the reciprocal of highest dilution showing 50% mortality in the inoculated mice and expressed as LD50 /ml and can calculated by suing either Reed-Muench or Karber formula. As an example, titration of rabies virus is illustrated in this section.
Materials :
1. Microbiological safety cabinet.
2. Sterile Pipettes, 10ml and 1ml
3. A tray with ice flasks. Rabies virus suspension (cell culture supernatant of infected brain emulsion)
4. Mice (Swiss albino or Laka) 4-6 weeks old
5. One ml syringe and needle for I/c inoculation
6. Test tubes.
7. Sterile PBS pH 7.4
8. Sterile bovine or equine serum
9. Cages for housing mice.
Procedure:
1. Working in microbiological safety cabinet, prepare the diluting fluid which is PBS containing 2% serum and dispense 9ml in test tubes labelled 10-1 to 10-7 and keep the test tubes in rack immersed in plenty of ice.
2. To make 10 fold (log) dilutions of the virus material, dilute 1ml of virus in 9ml of diluent to get the initial dilution i.e. 10-1. Subsequently transfer 1ml of previous virus dilution to next dilution by using at each step a fresh pipette, to achieve serial tenfold dilutions.
3.Inocultate 0.03 ml of each virus dilution intracerebrally into mice, starting from the highest dilution (in this case 10 -7). Use at least 6 mice per dilution and transfer these into cages appropriately labelled.
4.Observe the mice for 14 days. Any death occurring within first 5 days should be considered non-specific. Observe for specific signs and symptoms of rabies i.e., ruffling of hair, incoordination, tremors and paralysis of hind and fore limbs and finally death. Note down total number of specific deaths in each dilution and calculate the virus titre by using Reed-Muench or Karber formula.
Microtitration of Poliovirus using Vero cell line
Most of the commonly encountered human viruses produce characteristic cytopathic effect in one or the other cell lines routinely used in virology laboratories. The infectivity titres of these viruses can conveniently be determined by infecting a particular cell line with increasing dilutions of the virus material and determining the highest dilution producing cytopathic effect in 50% of the inoculated cells. The 50% end point dilution which in this case is expressed as TCID 50/ml can be calculated by using either Reed-Muench or Karber formulae. As an example, titration of polio virus I is illustrated in this section.
Materials :
1) Microbiological safety cabinet
2) Sterile test tubes or Pencillin vials
3) Micro pippettes, 200 & 1000 microns
4) Co2 incubator.
5) 96 well flat bottom tissue culture plates(Nunc)
6) Minimum Essential Medium(MEM,Sigma,HI) supplemented with antibiotics
7) Fetal calf serum(FCS)
8) Trypsin versene glucose coln.(TVG)
9) VERO cells.
10) Fluid containing PI.
11) Inverted microscope
Procedure :
1.Make 10 fold serial dilutions, say from 10-3 to 10-7 of the virus material in MEM containing 2% FCS, dispensed in either test tubes or penicillin vials, changing pipettes or tips with each dilution. Keep the tubes in rack immersed in ice.
2. Transfer 100 ul of each dilution to 4 wells of the micro-titre plate, starting from highest dilution to the lowest.
3. Trypsinise one MD bottle containing a confluent monolayer of Vero cells and count the cells and dilute to 4x 105 cells per ml. In MEM containing 10% FCS. Dispense 100 ul of cell suspension in to each of the well containing virus dilution and also include 4 wells as cell control in which 100Ul of cell suspension is mixed with 100 UL of MEM with 2% FCS. While dispensing the virus dilutions and cell suspension it is necessary to keep the plate on ice tray.
4. Cover the plate and keep it in CO2 incubator and adjust the temperature to 37 C.
5. Read the plate under an inverted microscope after 3 to 4 days when a confluent monolayer of Vero cells can be seen in control wells. Look for the cytopathic effect in the wells inoculated with virus dilutions. This consists of rounding of cells, i.e. if 2 of 4 wells inoculated with 10-8 dilution shows cytopathic effect then the titre is 10-8 per 0.1 ml. The titre can also be calculated by the method of Reed and Muench.
Calculation of 50% endpoints
In any biological quantitation, the most desirable endpoint is one representing a situation in which half of the inoculated animals or cells show the reaction (death in the case of animals and in CPE case of cells) and the other half do not. In other words, the endpoint is taken as the highest dilution of the biological material , which produces desired reaction in 50% of the animals or cells. The 50% endpoint can be based on several types of reactions. The most widely used endpoint, based on mortality, is the LD50 (50% lethal dose). This terminology can also be applied to other host systems-for example, tissue cultures - in which the TCID50 represents the dose that gives rise to cytopathic effect in 50% of inoculated cultures. When computing, if closely-placed dilutions are used and in each dilution large number of animals or cells are used, it may be possible to interpolate a correct 50% end point dilution, but it is neither practical nor economical. Reed and Muench devised a simple method for estimation of 50% endpoints based on the large total number of animals, which gives the effect of using at the 2 critical dilutions between which the endpoint lies, larger groups of animals than were actually included in these dilutions.
Calculation of the LD 50 titre by the Reed-Muench method
Let us presume that we have titrated a virus suspension by inoculating mice and death is the reaction. The number of deaths and survivals in each dilution is tabulated as given in table I below:
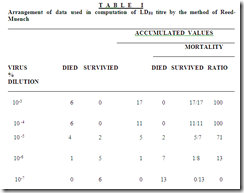
In the example depicted in the table it can be seen that mortality in the 10-5, is higher than 50% and in the next higher dilution, 10-6 it is only 13%. So, we need to find the 50% endpoint dilution , which obviously lies between these dilutions. First, we have to calculate the proportionate distance (PD) of the 50% endpoint from these dilutions by using a simple formula.

Negative logarithm of LD50 titre = (negative logarithm of the next dilution above 50% mortality + PD) x dilution factor i.e. in this example, (-5 + 0.4) x 1 =
-5.4 or log LD50 titre = 10-5.4 per 0.03 ml which is the amount of material inoculated I/C. The same methodology can also be applied to compute TCID50 when cell culture system is used for virus titration. Each dilution is inoculated into a minimum of 4 to 6Wells in a 96 well tissue culture plate and observed for CPE , which in this case replaces mortality.
Virus titration by using the plaque assay
1. Prepare confluent monolayers of cells (Porcine kidney for JEV and Hep-2 for Polio virus) in 24 well plates (Nunc).
2. Prepare serial 10-fold dilutions (101 to 107 ) of virus in chilled maintenance medium (MEM, with 1% serum).
3. Remove culture medium and add 0.2ml of virus inoculum, starting from the highest dilution. Ensure that a film of medium completely covers the cell sheet.
4. Incubate the plate at 37° C for 1 hour with intermittent rocking of the plate.
5. Remove the inoculum, preferably with a pipette and then add 1.5 ml of agarose overlay medium (growth medium with 0.3% agarose and 2.5% FCS).
6. Ensure that the overlay medium has spread evenly over the monolayer, leave at room temperature for 10 mins and then incubate at 37° C .
7. Examine the monolayers daily, starting from second day of incubation.
8. Once the plaques have developed, usually by the fourth day post inoculation, count the number of plaques at each dilution, remove the agarose overlay and gently wash the monolayer with PBS.
9. Stain the plate with 0.1% crystal violet solution and count the plaques again.
10. Estimate the virus titre as a plaque forming units per ml (pfu /ml) as follows by counting the number of plaques at an appropriate dilution.
For example :
Number of plaques produced 9
Dilution of virus 1 x 105
Volume of inoculum 0.2 ml
Virus titre = 9 x 1x105 x 1/5 pfu per ml
= 4.5 x 106
V ISOLATION AND IDENTIFICATION OF ENTEROVIRUSES FROM CLINICAL SPECIMENS
Enteroviruses including polio viruses cause a variety of CNS manifestations including aseptic maningitis, encephalitis and paralysis. These viruses are natural inhabitants of human intestinal tract and they can be easily isolated from samples of feces or from rectal swabs. But mere isolation of a enterovirus from stool sample may not prove the diagnosis as these may be present in normal stool specimens also. Therefore isolation should also follow a demonstration of a serological response to corroborate the diagnosis. However isolation of virus from CSF, or from brain postmortem cases is diagnostic.
Isolation of Enteroviruses from fecal specimens:
Materials:
1. 4-8 gms of feces,
2. 2. Centrifuge tubes.
3. 3.Cryo centrifuge
4. 4. Test tubes
5. 5.Rubber corks size 2,3 5.Micro pipettes
6. 6.CO2 incubator
7. 7. Water bath
8. 8.MEM with antibiotics and FCS 10% and 2%
9. 9.Hep 2 cells (ATCC, CCL 23)
10. 10. RD cells ( ATCC< CCL 136)
11. TVG soln.
12. 96 well flat bottom tissue culture plates.
13. 13.LBM pools of Antisera.
Processing of fecal specimens :
About 4-8 gms of feces is required. The specimens are kept frozen or in cold till processed. A 10% or 20% fecal suspension is made in cold sterile PBS in a tightly stoppered bottle. After vigorous shaking to emulsify the feces, the specimen is allowed to settle in the cold for 10min. The supernatant fluid is then poured in to a centrifuge tube and clarified at 3000 rpm for 10min. The supernatant fluid is removed and to this is added antibiotics to get a final concentration of 1000 units of penicillin and 1000 ug of streptomycin per ml and the sample is held at room temperature for 30min. It is then centrifuged in cold at speed 10,000 rpm for 20 min. The clear supernatant is separated and used immediately or stored frozen.
Inoculation of specimens into cell culture:
For isolation of most enteroviruses a combination of 2 cell lines like Hep 2 and RD will suffice. These cells are grown separately in test tubes and monolayers are obtained by employing usual cell culture techniques, described elsewhere. The sample processed is inoculated in 200 Ul quantities to each of 4 tubes of both Hep 2 and RD cell lines containing 1 ml maintenance medium and incubated in an almost horizontal position at 37 ° C . Cultures showing early degeneration, less than 24hrs are subpassaged. The tubes are observed daily for evidence of typical enterovirus CPE and discarded at the end of 7-10 days if found negative.
Identification of Enterovirus by Neutralisation : Ex: Poliovirus
If typical CPE is seen with either Hep 2 or RD or both cell lines, it is almost certain that an enterovirus has been isolated. The isolate may be identified as one of the polio antibodies. The isolate should be titrated and a dilution containing 100 TCID 50 per 0.1 ml is prepared. Each of the polio antisera P1, P2 and P3 is diluted to contain 50 antibody units, i.e.50 times that necessary to neutralise 100 TCID 50 of virus. Equal quantities of virus and serum are mixed and incubated in water bath at 37 C for 2hrs. The mixture is then inoculated on to monolayers of HEP 2 or RD cell lines in test tubes or microtitre plates. The reading is taken on day 3 and 7 and if the isolate is one of the poliovirus it will be neutralised by the corresponding antisera and hence there will be no CPE in the inoculated cultures. Virus controls not treated with antisera should show typical CPE at the end of 3rd day. Enteroviruses, which produce CPE, can be conveniently typed by neutralization using LBM pool (Lim-Benyesh-Melnick) of antisera. For further details about this technique as well as interpretation of results please refer to Melnick et al (1979). Enteroviruses. In : Diagnostic procedures for viral, Ricketsial and Chlamydial infections (E.H.Lennette, N.J.Schmitt, Eds)
5th edition, pp 471 - 524. American Public Health Association, Washington,USA.
Isolation of virus from CSF :
Enteroviruses can sometimes be isolated from CSF during early stages of the illness. For isolation from CSF 200 Ul of CSF is inoculated on to the monolayers of both Hep 2 and RD cells as described previously. No pre-treatment or addition of antibiotics is required. The tubes are incubated and observed for CPE as in case of fecal specimens. If an isolate is obtained it is identified as described earlier.
VI IMMUNOASSAYS FOR DETECTION OF ANTI-VIRAL ANTIBODIES
Haemagglutination inhibition (HI) test
A wide variety of animal viruses have been shown to possess the capacity of adsorbing to red blood cells and in many cases causing agglutination of these cells. This haemagglutination (HA) test furnishes a relatively simple, quick, convenient and fairly quantitative way of detecting , identifying and titrating viruses.
Virus haemagglutination is readily inhibited by specific antibody and the haemagglutination inhibition test furnishes a delicate way of detecting viral antibody. It may be strain-specific as in the case of the influenza viruses or group-specific as with most of the arboviruses where the test is mainly used for antigenic group screening.
Materials required :
Reagents:
1. 25% kaolin borate saline pH 9.0.
2. 0.4% bovine albumin in borate saline
pH 9.0 (0.4% BABS pH 9.0).
3. Virus adjusting diluents of varying pH (VADs)
4. Goose erythrocytes (RBC) in DGV
5. Antisera
6. Antigens
7. Normal serum
8. Acid citrate dextrose (ACD)
9. Dextrose gelatin veronal (DGV)
10. Borate saline pH 9.0
11. 0.9% NaCl
12. Acetone
13. 2-Mercaptoethanol
Preparation of Reagents
1. Borate saline pH 9.0
1.5 M NaCl 160 ml
0.5 M H3BO4 (Boric acid) 200 ml
1.0 M NaOH 47 ml
Make it to 2 litres with D/W, pH adjusted to 9 with 1M NaOH or 0.5M H3BO3.
2. 0.4% Bovine albumin in 1000ml borate saline pH 9.0 kept O/N in the cold.
pH…………………..8,6
Adjust pH 9.0 with 1M NaOH.
3. 25% kaolin in 100 ml borate saline pH 9.0 (Use acid-washed kaolin)
Borate saline is stirred on a magnetic stirrer and kaolin powder is added slowly to make a uniform suspension.
4. Dextrose-gelatin-veronal (DGV) for preservation of goose cells:
A. Gelatin 0.600 g
Diethyl barbituric acid (Veronal) 0.580 g
B. Sodium barbitone 0.380 g
CaCl2 (Anhydrous) 0.020 g
NaCl 8.500 g
Dextrose 10.000 g
Merthiolate (Thiomerosal powder) 0.100 g
MgSO4.7H2O 0.120 g
Add 250 ml of hot D/W to A and 750 ml of D/W to B. Mix A and B.
Autoclave at 10 lbs for 10 minutes.
5. 0.9% NaCl
9 g NaCl in 100 ml D/W. Autoclave at 15 lbs for 15 minutes.
6. Preparation of stock solutions
A. 1.5M sodium chloride (NaCl) - 87.675 g NaCl made to 1 litre with distilled water.
B. 0.5M boric acid (H3BO3) - 30.92 g H3BO3 are dissolved in about 700 ml of hot D/W. The solution is allowed to cool to room temperature and then made to 1 litre with D/W.
C. Concentrated sodium hydroxide (NaOH) - approximately 500 ml of distilled water is added to approximately 500g of NaOH pellets. The concentrated NaOH is about 18M and is very stable.
D. 0.5M dibasic sodium phosphate (Na2HPO4) - 70.99 g Na2HPO4 (anhydrous) made to 1 litre with distilled water.
E. 1M monobasic sodium phosphate (NaH2PO4) - 138.01 g NaH2PO4.H2O made to 1 litre with distilled water.
Collection/washing of goose RBC
a. Collection of RBC
1. Goose RBC are collected under sterile condition.
Take 125 ml of DGV in a 250 ml flask.
2. Take 10 ml ACD in a sterile tube.
3. Withdraw 7.5 ml ACD into 50 ml syringe fitted with a 20 gauge needle.
4. Collect 50 ml goose blood from the wing of jugular vein.
5. Remove needle from syringe and transfer blood from syringe to flask with DGV.
6. Mix well.
7. Store at 4°C (can be kept for up to 2 weeks)
b. Washing of RBC
1. Centrifuge required quantity of RBC suspension in a graduated centrifuge tube for six
Minutes at 2000 rpm.
2. Aspirate off the pipette attached to a rubber bulb without disturbing the RBC.
3. Add 2 ml of normal saline (NS).
4. Mix gently with Pasteur pipette to resuspend cells and add an extra quantity of NS to bring to the original volume.
5. Centrifuge at room temperature for 3 minutes at 200 rpm.
6. Repeat steps 2,3,4 & 5.
7. Centrifuge at 2000 rpm for 6 minutes.
8. Read volume of packed RBC.
9. Remove supernatant fluid.
10. Add 0.9% NaCl to make a 10% suspension of RBC and mix. The cells are ready for use in IV (use washed and packed cells for adsorption of sera).
Titration of Antigen
1. With a wax pencil mark a "U" microtitre plate as shown in figure:
20 40 80 160 320 640 1280 2560 5120 10240 cc** cc
6.0
6.2
6.4
6.6
6.8
7.0
7.2
· cc - cell control.
2. With a 0.05 ml dropper, add one drop of 0.4% BABS pH 9.0 to each well.
3. Keep plate in refrigerator for chilling.
4. Keep seven 0.05 ml micro diluters with tips immersed in 0.9% NaCl in a refrigerator for chilling.
5. Dispense 0.9 ml of 0.4% BABS in a tube in an ice bath.
6. Add 0.1 ml of antigen and mix thoroughly (1:10 dilution of antigen).
7. Remove plate and micro dilutor from refrigerator.
8. Blot 0.05 ml micro dilutors on a blotting paper, dip the tips in tube with 1:10 antigen and place in each of the wells marked 1:20 in the plate.
9. With the micro dilutors, prepare serial 2 - fold dilution of the antigen upto well 10 (1:10,240) by swirling the micro dilutors 10 to 15 times.
10. Add 2.4 ml of VADs 6.0, 6.2, 6.4, 6.6, 6.8, 7.0, & 7.2 to labelled tubes.
11. Add 0.1 ml of 10% RBC to each of the tubes and mix well (this gives a dilution of 0.4% RBC).
12. With a 0.05 ml dropper pipette add one drop of RBC to the corresponding row.
13. Tap plate gently to mix cells and the antigen.
14. Cover the plate and incubate at room temperature until cells in the wells of the cell control row settle down and form a button (usually this takes 20 - 30 minutes)
15. Read and record the titration.
16. Complete agglutination consists of a uniform layer on the lower surface of the well. A negative consists of a compact, sharply demarcated button of sedimented cells in the centre of the well, the pattern being identical to that seen in the control wells. The results are coded as follows:
20 40 80 160 320 640 1280 2560
+ + + + + + 0 0
then the antigen titre is the average of the antigen dilutions 640 and 1280 i.e.
(640 + 1280) /2 = 960.
17. The VAD with which the antigen shows the highest titre is the optimum one and should be used for that particular antigen.
18. Determine the dilution, which will contain 8 units per 0.025 ml by dividing the endpoint by 16. For e.g., end point 1280 = (1280/16) = 80. Therefore a 1:80 dilution of the antigen will contain 8 HA units per 0.025 ml.
19. Calculate the quantity of antigen required for the test and dilute the antigen accordingly.
20. Store antigen at 4°C.
Treatment of sera for HI test
The sera may be either extracted with acetone or treated with kaolin. These sera are then adsorbed
With goose erythrocytes to remove heamagglutinins for goose erythrocytes.
a Acetone extraction of sera
1. Label 16x120 mm tubes with specimen number of sera (this should include negative and
positive sera).
2. Add 0.9 ml normal saline to each tube.
3. Add 0.1 ml serum to each correspondingly labelled tube.
4. Keep sera in an ice bath.
5. Add 12 ml chilled acetone to each tube.
6. Stopper each tube and mix by inverting tubes several times.
7. Incubate for 5 minutes mixing occasionally.
8. Centrifuge at 3000 rpm for 5 minutes at 4°C.
9. Decant supernatant fluid.
10. Add 12 ml fresh acetone to each tube.
11. Mix thoroughly.
12. Incubate in cold for 1 hour. Mix the material occasionally.
13. Centrifuge at 4°C for 5 minutes at 2000 rpm.
14. Aspirate off the supernatant.
15. Tap each tube against palm of hand so that sediment forms a uniformly thin layer.
16. Desiccate in a desiccator attached to a vacuum pump for 1-2 hours.
17. Add 0.5 ml borate saline pH 9.0 to each tube and mix until completely resuspended (keep for minimum of 1 hour in cold).
18. Adsorb with goose cells.
a. Kaolin treatment of sera
1. Label 12x75 mm tube with specimen numbers of sera.
2. Add 0.1 ml serum to each correspondingly labelled tube.
3. Add 0.4 ml of kaolin solution (25% in borate saline, pH 9.0), to each tube and mix by shaking tube rack.
4. Incubate for 20 minutes at room temp. shaking every 5 minutes.
5. Centrifuge at 2500 rpm for 30 minutes at room temp.
6. Adsorb with goose cells.
b. Adsorption of sera with goose cells
1. Keep sera (acetone extracted or kaolin treated) an ice bath.
2. Add 0.1 ml of washed and packed goose RBC to each tube (It may be necessary to add more
cells for pig and rabbit sera)
3. Mix well and incubate for 20 minutes in an ice bath, mixing every 5 minutes.
4. Centrifuge at 2000 rpm for 15 minutes at 4°C.
5. Transfer supernatant fluid to fresh tube by decanting (transfer labels at the same time).
The sera are ready to be tested in HI.
Preliminary titration of antigen
The diluted antigens are titrated again before using them in the test.
1. Mark a "U" type microtitre plate as shown below.
Antigen Dilution Cell
Number ------------------------------------------------------------ control
1 2 3 4 5 6 7
1
2
3
4
2. Add a drop of 0.4% BABS pH 9.0 to each well from dilutions 1-7 and cell controls in rows 1-5
with a 0.05 ml dropper.
3. Keep plate in the refrigerator for chilling.
4. Keep 5 microdiluters (0.05 ml) with tips immersed in 0.9% NaCl for chilling.
5.Blot out the tip of each microdiluter. Dip the tip in each of the antigens and place in each of
the corresponding well dilution no.1.
6. Prepare serial two-fold dilutions of each antigen from well No.1 to well No…
7. Add one drop of 0.4% cells in required VAD with a 0.05 ml dropper pipette to corresponding
rows of antigens including cell control wells.
8. Shake the plate gently and incubate at room temperature till cell controls settle down
(about 30 minutes)
9. Read and record the titration. Adjust if necessary and re-titrate.
Performance of HI test
1. Select antigens to which sera are to be tested.
2. Treat all test sera, antisera homologous to antigens selected and a negative control serum, as shown in treatment of sera.
3. Mark the "U" type microtitre plates to accommodate the number of sera to be tested for each antigen. Also mark the control plate (see below)
4. With a 0.025 ml dropper add a drop of 0.4% BABS pH 9.0 to each well.
5. Dip pretested 0.025 ml micro diluters into each treated serum and place in well No.1
(1:10 dilution).
6. Prepare serial two-fold dilutions with the microdiluters for each antigen and for serum
controls.
7. With a 0.025 ml dropper add a drop of test virus antigens containing 8 HI units, to all Wells of the serum dilutions in each test plate.
8. With a 0.025 ml dropper add a drop of 0.4% BABS pH 9.0 to all wells of the serum dilution series in the controls plate. Shake plates to mix, cover and keep in the refrigerator.
9. Mark the plate for post incubation titration.
10. Add 0.05 ml of 0.4% BABS pH 9.0 to each well with dropper.
11. Add 0.05 ml of each antigen to the first well and mix.
12. Incubate all plates at 4° C overnight (about 18 hours)
13. Place a 0.05 ml microdiluter in each of the sells containing antigen diluent mixture in
The post incubating titration plate (well No.1).
14. Prepare serial two-fold dilutions through well No.7.
15. Add one drop of 0.4% goose RBC in respective VAD to each well of titration plate with 0.05ml dropper.
16. Shake and incubate at room temperature.
17. Add one drop 0.4% goose RBC (in respective VAD) to each well of test plates and control plates with a 0.05 ml dropper.
18. Shake plates and incubate at room temperature till the cells in controls settle down.
19. Read and record the cell patterns.
a. Check RBC controls. These should settle down into a button.
b. Check the titration of antigens. Each antigen should contain 4-8 HA units.
c. Check the serum controls. If no agglutination occurs in any dilution of a serum the HI results in test plates are considered valid.
d. Check the negative control serum. It should show complete agglutination in all dilutions. If not, the test is invalid.
e. Check the positive reference serum. The serum must show complete inhibition of
haemagglutination.
f. Check the test sera. The end point used to designate the titre of HI antibody
present is the highest dilution of serum that inhibits heamagglutination.
Early convalescent sera are treated with 2-ME to detect the presence of IgM antibodies. The acetone extracted sera are treated with 2-ME. Prepare 1M 2-ME in borate saline pH 9.0 : (a) 1.47 ml 2-ME +18.53ml or (b) 1.56 ml + 18.44 ml, depending upon the molecular weight of stock solution. Add one part of 2-ME (1M) to four parts of serum and incubate the mixture for one hour at 37° C and make serum dilutions. For post titration of antigen with 2-ME, 0.2M 2-ME is mixed with equal volume of antigen and incubate overnight at 4° . Antigen dilution is carried out and HA titre is determined. The 2-ME treated and untreated sera are tested in HI as per routine test.
VIRUS NEUTRALISATION TESTS
A virus will lose its infectivity when it is mixed with specific antiserum raised against that virus. This is called neutralisation. The neutralised virus is unable to infect any cell culture or experimental animal. This property of the virus can be conveniently employed for its identification and also for detection and quantification of specific antiviral antibodies. In the virus neutralisation tests for antibody assay, a fixed amount of a specific virus is made to react with increasing dilutions of a specific antiserum under standard conditions and the degree of virus neutralisation is determined either in vivo by animal inoculation or in vitro by infectivity in cell culture. On the other hand, for identification of a particular viral isolate, a fixed quantity of virus is reacted with a standard amount of specific antiserum and also with normal serum. The identity of the virus gets established if it is neutralised by specific antiserum and remains infectious with normal serum.
In this section we will illustrate assay of anti-rabies serum by in vivo neutralisation in mice and detection and quantification of polio virus antibodies by in vitro neutralisation in cell culture.
ASSAY OF ANTIRABIES SERUM BY MOUSE NEUTRALISATION TEST (MNT)
This test is the standard test recommended for estimation of anti-rabies antibodies in human or animal sera, to know the level of protection after vaccination of for diagnosis of the disease. In this test different dilutions of the serum under test is incubated with a standard amount of a fixed rabies virus strain known as CVS (challenge the virus strain) and serum virus mixture is inoculated I/C in to weanling mice and depending on the survival rate in mice the titre can be expressed in international units (IU) in comparison to a standard anti-rabies serum whose unitage is known.
MATERIALS REQUIRED :
1.Sterile test tubes or penicillin vials.
2.Pipettes, 10 ml, 5 ml and 1 ml
3.Water bath
4.1 ml syringes
5. Weanling mice, 4 weeks old
6. Sterile PBS pH 7.4
7. Bovine or horse serum
8. CVS of known titre. 9.Reference ARS of known unitage.
PROCEDURE :
1. Inactivate sera under test at 56 C for 30 min in water bath.
2. Prepare dilutions of test and reference sera in PBS containing 2% bovine of horse serum. Dilutions should be so adjusted as to get 50% end points preferably in the middle dilutions. To get a dilution of say 1:8 add 0.1 ml of serum to 0.7 ml of diluent and then continue with doubling dilutions i.e 16,32,64 etc.
3. Prepare a dilution of CVS containing 100 LD50/0.03 ml so that when mixed with an equal quantity of serum dilution the challenge is reduced to 50 LD50. To calculate the titre of CVS giving 100 LD 50 we should know the original titre of CVS. For E.g. if the titre is 10-7 /0.03 ml then 100 LD50 will be present in dilution 10-7 / 0.03 ml then 100 LD50 will be present in dilution 10-5.
4. Mix 0.5 ml of serum dilution with 0.5 ml of CVS dilution, 10-5 and incubate in a water
bath at 37° C for 60 min. Simultaneously prepare 3 more dilutions of CVS i.e, 10-6 to 10-8and mix 0.5 ml of this dilution with 0.5 ml of diluent. This is also incubated along with serum-virus dilutions.
5. After 60 min remove the tubes from water bath and chill the tubes by keeping in
Refrigerator at 4° C or in a ice tray.
6. Inoculate I/C 6-8 mice with each serum dilution and to determine the Exact LD50 used for challenge inoculate virus dilutions also in similar manner. Observe the mice for 14 days. Ignore all deaths before 5 days post inoculation. Note down the specific deaths occurring from 6 to 14 days.
7. The 50% end point titre of serum called effective dose 50 (ED50) or neutralising dose 50
(ND50) is calculated by Reed and Muench method. At the same time note down the mortality in mice inoculated with CVS dilutions and calculate the exact LD50 used in the test. This should be approximately 50 LD50.
8. The titre of the test serum can be expressed in IU/ml in comparison to reference serum. E.g. If reference serum has an IU of 5 IU and its ND50 is 1:128 and the test serum shows ND50 of 1:64 then the unitage of test serum is
reciprocal of
test serum ND50
_____________________________ X unitage of reference serum
reciprocal of
reference serum ND50
i.e.
64
______ X 5 = 2.5 IU/ml
128
ASSAY OF POLIO VIRUS ANTIBODIES BY NEUTRALISATION TEST IN VITRO BY USING CELL CULTURE TECHNIQUE
Antibodies to polio viruses develop after vaccination with polio vaccine or after infection with wild virus. Antibodies to polio and other enteroviruses may be present in normal population and hence demonstration of four fold rise in the titre of antibody is absolutely necessary for the sero- diagnosis of enteroviral infections. For the test, two serum samples, one taken just after the onset of the disease and the second, after a gap of 2 weeks or more is required.
In the test proper, increasing dilutions of patient's serum is mixed with 100 LD50. Of the 3 types of polio viruses and incubated in water bath for 2 hrs. The mixture is then inoculated to preformed monolayers of either Vero or Hep 2 cells in flat bottom 96 well tissue culture plates and incubated for 7 days. Readings are taken at 3rd and 7th day and depending on the CPE seen in different dilutions, titre of the serum sample is calculated by using Reed and Muench method.
MATERIALS REQUIRED :
1. 96 well flat bottom tissue culture plates
2. Micro pipettes, 200 and 1000 Ul
3. Microbiological safety cabinet (Laminar flow)
4. CO2 incubator.
5. Test tubes or penicillin vials
6. MEM with added antibiotics and containing 2% FCS
7. Water bath 56° C and 37° C
PROCEDURE :
Each serum sample has to be tested separately for antibodies against 3 polio viruses. Before starting the procedure a plan should be drawn for the distribution of samples and controls in the microtitre plates.
1. Inactivate the serum samples at 56 C for min.
2. Dilute the serum samples from 1:16 to 1:2048 in test tubes or penicillin vials using MEM with 2% FCS.
3. Dilute the 3 types of polio viruses, P1, P2 and P3 in MEM to contain 100 TCID 50 per
0.1 ml using MEM with 2% FCS.
4. Mix equal serum quantities of serum dilution and virus dilution in test tubes and incubate in
water bath at 37 C for 2 hrs.
5. Simultaneously prepare three 10 fold dilutions of challenge virus dose starting from the
dilution containing 100 TCID 50. Mix equal quantities of these dilutions and MEM with 2% FCS and incubate in a similar manner.
6. At the end of incubation, dispense 100 ul of serum virus mixture and virus MEM mixture to
wells of the micro titre plate, starting from lower dilution of the serum to the higher. Include 2 wells for cell control and to this add 100 UL of MEM. Each serum dilution and virus dilution should be inoculated to at least 4 wells.
7. Incubate the plates in a CO2 incubator and take the reading at the end of 3rd and 7th day. Look
for the CPE in wells in which virus dilutions were added and calculate the actual TCID50
employed in the test. This should be around 100 TCID50 per 0.1 ml. Note down the highest serum dilution showing complete neutralisation of CPE. Calculate the 50% endpoint dilution by using Reed -Muench formula. If there is initial neutralisation at day 3 followed by breakthrough at day 7, it indicates partial neutralisation due to antigenic relation with other enteroviruses.
ENZYME LINKED IMMUNOSORBENT ASSAY
Preparation of solutions and reagents for ELISA
1. 0.05M carbonate buffer pH 9.6.
- Dissolve 0.53 g Na2CO3 (anhydrous) in distilled water and make final volume to 100 ml.
- Dissolve 0.42 g NaHCO3 in distilled water and make final volume to 100 ml.
To 32 ml of solution A, slowly B till pH is 9.6 (approximately 68 ml).
2. Phosphate citrate buffer, pH 5.0.
a. Dissolve 11.9 g of Na2HPO4, 2H2O in distilled water and make up to 1 litre (Solution A).
b. Dissolve 7.0 g of citric acid in distilled water and make up to 1 litre (Solution B).
Mix 55 ml of Solution A with 45 ml Solution B.
Adjust pH to 5.0 with either a or b if necessary.
3. 0.01 M Phosphate buffer saline (PBS), pH 7.2.
Dissolve 8.0 g of NaCl, 0.2g of KC1, o.2g of KH2PO4 and 1.5 g of Na2HPO4. 2H2O in distilled water and bring the final volume to 1000ml. Adjust pH to 7.2 with 0.1 N NaOH or 0.1N HC1 if necessary. Add 0.5 ml/L Tween 20 (Polyoxyethylene sorbitan monolaurate) to 1X PBS to make PBST.
ANTIGEN PREPARATION OF HERPES SIMPLEX VIRUS
1) 0.5ml of a stock of Herpes simplex virus (HSV-1) is added to a Roux bottle with a monolayer of Vero cell line after discarding the growth medium.
2) Adsorb the virus for 30 mins at 37° C and add 100 ml of maintenance medium. Incubate in a CO2 incubator at 37° C.
3) Observe for evidence of CPE (24-48 h).
4) Remove the supernatant medium and harvest the cells with 10-20 ml of sterile PBS, when >90% of the cells show CPE.
5) Centrifuge for 5 mins at 1000 rpm and wash the cells thrice with sterile PBS.
6) Resuspend the cells in a minimal amount of PBS (ex:1ml).
7) Sonicate the suspension at 60 cycles/second continuously for 5 mins and pulsed sonication for 3 mins.
8) Centrifuge the sonicate for 10 mins at 1000 rpm.
9) The supernatant is used as the antigen after estimating the protein concentration.
10) Control Vero cell antigen is prepared in a similar manner with mock infected Vero cells. (Sterile medium to be used instead of the virus suspension).
NOTE : The protein values of the HSV and Vero cell antigens should be matched before it is used in the test.
MEASLES VIRUS ANTIGEN PREPARATION
1) 1 vial of commercial Measles vaccine reconstituted in 0.5 ml of cell culture medium and
made up to 5 ml with same medium and inoculated into 1 Roux bottle with a monolayer of Vero cell line.
2) Adsorption is carried at 37° C for 1 h
3) Maintenance medium is added.
4) Cytopathic effect is first seen on third day and it gradually increases over next four days. On the seventh day extensive CPE can be seen
5) The fluid is then frozen and thawed twice and kept as seed stock.
PREPARATION OF ANTIGEN
1. Virus is grown in Roux bottle and fluid harvested aseptically.
2. Spin at 1000 rpm for 30 min.
3. To supernatant add sodium chloride (4gms/100ml) and PEG precipitate at a concentration of 10%. (10gms/100ml); (add Nacl first allow it to dissolve and then add PEG slowly)
4. Stir for half an hour in ice bath and add 0.1% sodium azide while stirring.
5. Keep for two hours or overnight in refrigerator.
6. Spin at 10000 x g for 30 min.
7. Discard supernatant.
8. Resuspend pellet in 1/100th of the original volume with normal saline.
9. Dialyse against sterile normal saline containing 1% sodium azide for 24h with three changes. (Dialysis bags are pretreated by boiling in 1% sodium carbonate solution in distilled water containing lmM EDTA).
10. Collect the dialysed antigen centrifuge and collect supernatant 5MM EDTA + 200MM NaHCO3 in 100 ml D.W. x 20 min. and estimate the protein before using it in the test.
11. Control Vero antigen is prepared in a similar manner with fluid collected from mock infected Vero cells.
EXTRACTION OF JAPANESE ENCEPHALITIS VIRUS ANTIGEN WITH SUCROSE AND ACETONE
1. The infected suckling mouse brain is homogenised with four volumes of chilled 8.5%
aqueous solution of sucrose in homogeniser (Waring blender) (ex. If the total weight of the mouse brain is 2g then 8ml of sucrose solution should be added)
2. The homogenate is added drop wise with brisk mechanical stirring to 20 volumes of
chilled acetone. The mixture is shaken vigorously and allowed to stand for 15 min.
The milky supernatant acetone is decanted, the sediment at this stage being a pink gummy mass which is tightly adherent to the bottom of the centrifuge bottle.
3. A volume of fresh acetone equal to that originally used is added to each bottle and the
preparation are allowed to stand in an ice bath for at least one hour to dehydrate the
sediment; after sufficient time the sediment is readily reduced to a fine suspension by use of a thick glass rod. The bottles are then centrifuged as before. The supernatant fluid is
aspirated out and the sediments from all bottles are pooled into one bottle with the help of small quantity of fresh acetone.
4. After most of the final supernatant acetone has been removed by aspiration the bottle is
closed with a rubber stopper fitted with glass tubing, which is connected to a vacuum pump for drying. The drying is continued for one hour with the bottle kept immersed in an ice bath. The pump is protected from contamination by a trap containing a large quantity of non-absorbent cotton.
5.To the dry powder a volume of 0.9% NaCl is added which is equal to 2/5th of the total
Volume of homogenate originally used (Ex. If the homogenate is 10ml, 4ml of 0.9% NaCl is added for rehydration). The bottle with antigen is left overnight in ice water in the cold room. The next morning it is centrifuged at 10000 rpm for one hour. The supernatant fluid is antigen. For gel diffusion test uncentrifuged antigen is used.
Note : Tris buffer (0.1M) pH 8 to 9 can be used rehydration of sucrose acetone antigen instead of 0.9% NaCl.
Procedure for biotinylation of monoclonal antibody
Reagents :
1) Monoclonal antibodies : Ascitic fluids from BALB/c mice inoculated with desired clones have to be precipitated using the sodium sulphate technique. After dialysis the protein concentration is measured (absorbance at 280nm) and adjusted to 1mg in 0.1M sodium bicarbonate solution (stock solution).
2) Biotin-N-hydroxy succinamide (BRL Bethesda): 1mg of Biotin-n-hydroxy succinamide is dissolved in 1ml of dimethylsulfoxide (Sigma or Merck). Prepare it fresh on the day of use. DO NOT STORE SOLUTION PREPARED EARLIER.
3) Good quality dialysis bags (25000 mol.wt cutoff)
PROCEDURE
To 1000 ug of monoclonal antibody in 1ml of sodium bicarbonate solution, add 200 ul of stock biotin solution drop-wise (1mg/ml DMSO, it can be 100, 200 & 400 accordingly check before use). After mixing well incubate at room temp. for 4 hours with periodic mixing. At the end of 4 hours dialyse the mixture against sterile normal saline (500 ml x 3 changes) for 18 hours at 4° C to remove unbound biotin. After dialysis measure the exact volume. If the volume is 1 ml then the protein concentration is 500 ug/ml. Distribute 50 ul volumes into 20 Eppendorf tubes (bullets). Add 50 ul of sterile glycerol to each bullet (cryopreservative). Each bullet now contains 50 ug of biotinylated monoclonal antibody. Store at -70° C. Estimate optimal concentration of this diluent for this antibody. DO NOT USE PLAIN PBS SINCE HIGH BACKGROUND IS OBTAINED. The conjugate used is STREPTAVIDIN PEROXIDASE (BRC Bethesda) at a dilution of 1:1000 (50 ul/well in MAC-ELISA). Incubation with biotinylated monoclonal antibody is for 2 hours at 37° C in a water bath (float the plates).
DETECTION OF IgG ANTIBODIES TO HSV-1 BY ELISA
1. Coat ELISA plates with HSV and Vero antigens at a predetermined concentration in carbonate
buffer pH 9.6, 100 ul/well.
2. Incubate overnight at 4° C.
3. Discard and wash the plates thrice with PBST.
4. Quench the plates with 1% skimmed milk powder in PBST, 150 ul/well.
5. Incubate for 2hrs at 37° C.
6. Discard, wash plates thrice with PBST.
7. Add 100 ul/well sera, CSF appropriately diluted in PBST. Include positive, negative
controls and blanks.
8. Incubate for 2 hrs at 37° C.
9. Discard, wash thrice with PBST.
10. 100 ul/well of goat/rabbit anti-human IgG peroxidase conjugate (Bangalore Genei)
(dilution to be used: 1:2000 in PBST with 1% normal goat serum)
11. Incubate for 2 hrs at 37° C.
12. Discard, wash thrice with PBST.
13. Add 100 ul/well of a solution containing OPD (4mg/10ml of PCB, pH 5) with 10 ul
of H2O2.
14. Incubate in the DARK at room temperature for 20 minutes
15. Stop the reaction with 50 ul/well of 4N H2SO4.
16. Read the plate at 492 nm on an ELISA reader.
17. A sample is considered positive if the difference in OD values is >0.1 between the
HSV antigen and Vero antigen at that particular dilution. Antigen is considered
significant at that dilution.
DETECTION OF IgG ANTIBODIES TO MEASLES VIRUS BY ELISA
1. Coat ELISA plates (100 ul /well) with measles virus antigen and Vero antigen at a
predetermined concentration in carbonate buffer pH 9.6
2. Incubate overnight at 4°C.
3. Discard contents of the wells and wash thrice with PBST.
4. Quench with 1% skimmed milk powder in PBST (150 ul/well). Incubate at 37°C for 2 hrs.
5. Discard and wash thrice with PBST.
6. Add the required dilutions of CSF/Serum samples (100 ul/well) and incubate at 37° C for 2 hrs. Include positive , negative controls and blanks.
7. Discard and wash thrice with PBST.
8. Add 100 ul/well of rabbit anti-human IgG peroxidase conjugate (Bangalore Genei) diluted 1:2000 in PBST with 1% normal goat serum and incubate for 2 hrs. at 37° C.
9. Discard and wash thrice with PBST.
10. Add 100 ul/well of a solution containing OPD (4mg/10ml of PCB,pH5) and 10 ul of H2O2. Incubate at RT for 20 mins in the dark.
11. Stop the reaction with 50 ul/well of 4N H2SO4.
12. Read the results at 492 nm on an ELISA reader.
13. A sample is considered positive if the difference in OD values is > 0.1 between the measles virus antigen and Vero antigen at any given dilution.
DETECTION OF IgM ANTIBODIES TO JEV BY AN IGM ANTIBODY CAPTURE ELISA (MACELISA)
1. Coat ELISA plates with 100 ul/well of a predetermined concentration of rabbit anti-human IgM antibodies (DAKOPATTS) in carbonate buffer pH 9.6.
2. Incubate at 4° C overnight.
3. Discard and wash with PBST thrice.
4. Quench with 1% skimmed milk powder in PBST (150 ul/well). Incubate at 37°C for 2 hrs
5. Discard and wash thrice with PBST.
6. Add 100 ul/well of CSF (diluted 1:100 in PBST) in duplicates. Incubate for 4hr at 37° C.
7. Discard and wash with PBST thrice.
8. Add 100 ul/well of JEV mouse brain antigen (50HA units/100 ul diluted in PBS). Incubate at 4° C overnight.
9. Discard and wash with PBST thrice.
10. Add 100ul/well of biotinylated NIV cross-reactive monoclonal antibody to JEV (25ul/10 ml of PBST containing 1% skimmed milk powder and 1% normal goat serum). Incubate at 37° C for 2 hr.
11. Discard and wash with PBST thrice.
12. Add 100 ul/well of streptavidin peroxidase conjugate (DAKOPATTS) diluted 1:5000 in PBST containing 1% normal growth serum. Incubate for 15 mins at RT.
13. Add 100 ul/well of a solution containing OPD (4mg/10ml of PCB,pH5) and 10 ul of
H2O2. Incubate at RT for 20 mins in the dark.
14. Stop the reaction with 50 ul/well of 4N H2SO4.
15. Read the results at 492 nm on an ELISA reader.
16. Appropriate high positive control, low positive control, negative control for CSF and serum as well as blanks should be included in every assay.
17. The results are expressed as MACELISA units which is calculated using the following
formula :
ELISA units = (OD of test sample - OD of negative control)
--------------------------------------------------------- x 100
(OD of weak positive - OD of negative control)
Interpretation of results is as follows :
a. Any test sample with > 100 units is considered positive for IgM antibodies to JEV.
b. A sample with ELISA units between 30-99 is considered probably positive for IgM antibodies to JEV but requires further testing with Dengue and West Nile virus antigens as well as normal mouse brain antigen to exclude false positive reactions.
c. A sample with ELISA units < 30 is considered negative IgM antibodies to JEV.
IMMUNOASSAYS FOR THE DETECTION OF RETROVIRAL ANTIBODIES
Western blot analysis for HTLV-1 proteins
a) Preparation of Western blot strips : Use solubilized virus obtained from MT2 or HUT102 cells which has been purified and concentrated 1000 fold by sucrose gradient centrifugation .
b) Polyacrylamide Gel Electrophoresis :
1) Layer the 10% resolving gel and incubate for 15min. at RT with distilled water layered over it.
2) After this removing the water layer 4% stacking gel on to the resolving gel and insert combs into the stacking gel. Incubate at RT for 15 min.
3) Remove the comb and flush the wells with distilled water.
4) Carefully remove the clamps and mount the gel plates on to the Electrophoresis unit and add tank buffer into both lower and upper tanks. Ensure that the upper tank has sufficient buffer to cover the wells in the gel.
5) Denature the protein sample (HTLV-1 antigen) in sample buffer by boiling for 3 min. in a water bath. Load 25 to 50 ul of this denatured sample into each of the wells carefully avoiding spills and air bubbles using a micro pipette.
6) Run the gel in the constant voltage mode at a setting of 60 volts until the dye front reaches the bottom of the gel plate.
7) Remove the gels carefully and use for transferring of proteins to nitrocellulose membranes by Western Blotting.
c) Western Blotting :
1) Transfer Electrophoresed gel to tray containing Western Blot buffer (20% methanol in 1x tris-glycine buffer which has been de-aerated for 15 min.)
2) Place a pre-wetted nitrocellulose sheet, which has been cut to the same dimensions as the gel on the gel ensuring that there are no air bubbles trapped in between.
3) Keep filter paper pads below the gel and above the nitrocellulose paper to make a sandwich.
4) Place this sandwich in the appropriate clip and insert into the Electro blotting tank containing buffer.
5) Ensure that the anode is towards the nitrocellulose membrane side while the cathode is towards the gel.
6) The electroblotting is carried out using constant current mode at 150 MA for 2 hrs.
7) Remove the nitrocellulose membrane (always handle gently using a forceps) and place in a tray containing 1% milk powder in PBS. Incubate at 4° C overnight.
8) The nitrocellulose membrane is removed from the tray and carefully cut into thin strips. Store in a zip lock bag at 4° C for upto 1 month.
9) Place thin strips in a Western Blot immuno assay tray and wash with PBS using a rocking platform.
10) Add 2.5 ml of 1:50 dilution of CSF or 1:100 dilution of serum prepared in PBS containing 1% skimmed milk powder.
11) Incubate at room temperature on the rocking platform for 2 hrs. Wash thrice with PBST on the rocking platform.
12) Add 2.5 ml of appropriate dilution of anti-human IgG peroxidase conjugate prepared in PBST containing 1% milk powder and incubate on the rocking platform at RT for 2 hrs.
13) Wash thrice with PBST on the rocking platform.
14) Add 2ml of substrate solution (Diaminobenzidine 4 mg /10 ml of PBS and 10 ul of H2O2) and rock on the platform for 1 min. and keep the tray in the dark for 10 min. at room temperature.
15) Stop the reaction by adding distilled water to each of the wells.
ELISA procedure for detection of anti HTLV-1 antibodies
1. Use cell culture purified and concentrated virus solubilized virus for ELISA. The optimal
concentration should be pre-determined in a checkerboard titration. Usually 5-10 ug/ml
of viral antigen gives best results.
2. Coat plates with 100 ul of antigen diluted in 50 mM NaHCO3 buffer, pH 9.6 and incubate
at 4° C overnight.
3. Discard contents and rinse thrice with PBS. Add 200 ul of 2.5% BSA solution in PBS
containing 1.25 % milk powder and 5% sucrose to each of the wells and incubate at 37°
C for 2 hrs. Wash plates thrice with PBST.
5. Add 100 ul of CSF/serum samples diluted five fold (1:5 to 1:625) in PBST with 2% milk powder to the wells in the ELISA plate.
6. Incubate at 37° C for 2 hrs. Wash plates thrice with PBST.
7. Add 100 ul of goat anti-human IgG peroxidase conjugate (Bangalore Genei) diluted 1:1000 in PBST containing 1% normal goat serum. Incubate for 2hrs. at RT. Wash thrice in PBST.
8. Add 100 ul of substrate (4 mg % of OPD in PCB with 0.005% H2O2) and incubate in the dark for 20 mins. at RT.
9. Stop the reaction with 50 ul of 4N H2SO4.
10. Measure the OD values at 492nm in an ELISA Reader.


 11.55
11.55
 Rafless bencoolen
Rafless bencoolen





0 komentar:
Posting Komentar